Isotopes Worksheet
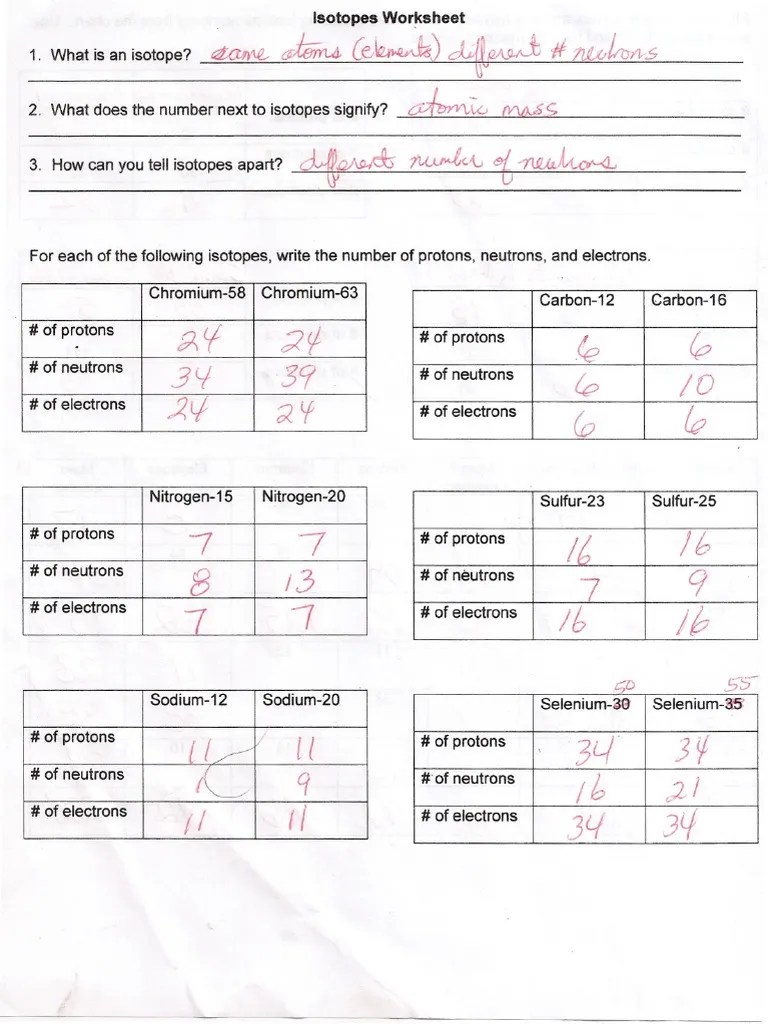
Isotopes Worksheet - What does the number next to isotopes signify? I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element The number 6 refers to the atomic number c. Worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides. How can you tell isotopes apart? Atomic # = # protons. Web isotope practice worksheet atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. The number indicates the isotope’s mass number. Free interactive exercises to practice online or download as pdf to print.
Web isotope practice worksheet 1. How many protons and neutrons are in the first isotope? What does the number next to isotopes signify? Worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides. How can you tell isotopes apart? The numbers 12, 13, and 14 refer to the mass number d. In a neutral atom, the number of positive protons equals the number of negative electrons.
I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element Describe the general arrangement of subatomic particles in the atom. How many protons and neutrons are in the first isotope? Web isotope practice worksheet 1. Web isotopes worksheets and online activities.
13 Best Images of Element Symbols Worksheet Answer Key Periodic Table
I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). The number indicates the isotope’s mass number. Isotopes are versions of.
5 Isotope Practice Worksheet
The number of protons in an atom determines the identity of the atom. There are naturally occurring isotopes and isotopes that are artificially produced. Web atoms and isotopes worksheet 1. Web isotopes worksheets and online activities. Use the periodic table to identify and count subatomic particles within the atom.
Isotopes Worksheet Answers
As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). Fill in the table with the correct information. # protons = # electrons. The number of protons in an atom determines the identity of the atom. Web isotopes worksheets and online.
Most Common Isotope Worksheet 1
They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. Web isotope practice worksheet atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. The numbers 12, 13, and 14 refer to the mass number d. Atomic # = #.
Isotopes Worksheet 1 Answer Key kidsworksheetfun
What does the number next to isotopes signify? The number of protons in an atom determines the identity of the atom. In a neutral atom, the number of positive protons equals the number of negative electrons. Use the periodic table to identify and count subatomic particles within the atom. How many protons and neutrons are in the second isotope?
Isotope Practice Worksheet
Fill in the table with the correct information. Web isotope practice worksheet name: How can you tell isotopes apart? What does the number next to isotopes signify? I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element
Unit 2 Chapters 4, 5 & 6 Mrs. Gingras' Chemistry Page
12c 6 13c 14c 6 6 a. What contribution did these scientists make to atomic models of the atom? The simplest example of an atom with different isotopes is hydrogen. Fill in the table with the correct information. Web isotopes worksheets and online activities.
Isotopes Ions And Atoms Worksheet 1 Answer Key —
Web isotope practice worksheet atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. How many protons and neutrons are in the second isotope? What does the number next to isotopes signify? I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for.
50 Isotopes Worksheet Answer Key
# protons = # electrons. They have the same number of protons and electrons as the element but different mass numbers and number of neutrons. I can use the atomic number of an element to identify protons and electrons for any element. Isotopes are versions of the same element. Web isotope practice worksheet atoms of a given element which have.
Isotopes Worksheet - Protons and neutrons both have a mass of 1 amu. Web isotope practice worksheet atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. Web isotopes worksheets and online activities. The number indicates the isotope’s mass number. Web here are three isotopes of an element: I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). How can you tell isotopes of the same element apart? Web isotope practice worksheet name: In a neutral atom, the number of positive protons equals the number of negative electrons.
I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element What does the number next to isotopes signify? Fill in the table with the correct information. The number 6 refers to the atomic number c. Web isotope practice worksheet 1.
Web isotope practice worksheet 1. The number indicates the isotope’s mass number. Web here are three isotopes of an element: There are naturally occurring isotopes and isotopes that are artificially produced.
What Does The Number Next To Isotopes Signify?
Atomic # = # protons. Isotopes are versions of the same element. What contribution did these scientists make to atomic models of the atom? Protons and neutrons both have a mass of 1 amu.
The Number Of Protons In An Atom Determines The Identity Of The Atom.
What does the number next to isotopes signify? The numbers 12, 13, and 14 refer to the mass number d. I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element Describe the general arrangement of subatomic particles in the atom.
12C 6 13C 14C 6 6 A.
The lesson on “atomic structure and notation” is provided free in my other resources, so you can try before you buy. Web atoms and isotopes worksheet 1. As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). There are naturally occurring isotopes and isotopes that are artificially produced.
Use The Periodic Table To Identify And Count Subatomic Particles Within The Atom.
How many protons and neutrons are in the second isotope? Fill in the table with the correct information. Web isotope practice worksheet 1. How can you tell isotopes of the same element apart?