Hesss Law Worksheet
Hesss Law Worksheet - Calculate l1h for the reaction: Web heat of formation worksheet. Hess's law is saying that if you convert reactants a into products b, the overall enthalpy change will be exactly the same whether you do it in one step or two steps or however many steps. \text {kj/mol}_ {rxn} kj/molrxn show calculator Web hess's law worksheet author: This means that the enthalpy change for the overall process will be identical regardless of. Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in one step or in a series of steps. Brooklyn city board of education C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: C 2h 4 (g) + 3 o 2 (g) à 2 co 2 (g) + 2 h 2o (l) δh = − 1411 kj / mole c 2h 6 (g) + 7/2 o 2 (g) à 2 co 2 (g) + 3 h 2o (l) δh = − 1560.
Web hess's law worksheet 1. Calculate δho for the reaction c2h4 (g) + h2 (g) → c2h6 (g), from the following data. Write your answer to the ones place. Web hess's law (worksheet) page id. Use a standard enthalpies of formation table to determine the change in enthalpy for each of these reactions. To use hess's law, two principles must be understood: Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in one step or in a series of steps.
Hess's law worksheet created date: Law of combining volumes (worksheet) For example, the enthalpy change for the reaction 2a + b → 2d can be calculated using the enthalpies of the two reference reactions: Calculate h for the reaction 4 nh 3 (g. C 2h 4 (g) + 3 o 2 (g) à 2 co 2 (g) + 2 h 2o (l) δh = − 1411 kj / mole c 2h 6 (g) + 7/2 o 2 (g) à 2 co 2 (g) + 3 h 2o (l) δh = − 1560.
Hess S Law Worksheet worksheet
Understand hess’s law and its use for calculating reaction enthalpies. \text {kj/mol}_ {rxn} kj/molrxn show calculator C 2h 4 (g) + 3 o 2 (g) à 2 co 2 (g) + 2 h 2o (l) δh = − 1411 kj / mole c 2h 6 (g) + 7/2 o 2 (g) à 2 co 2 (g) + 3 h 2o.
Hess Law Worksheet Pdf Thekidsworksheet
Calculate h for the reaction: So, if 100 units of energy are involved when p forms q i.e. C2h4 (g)+ h2 (g)~c 2 h 6 (g), from the following data. Kj / mole h 2 (g) + ½ o 2 (g) à h 2o (l) δh = − 285.8 kj / mole 2. Web hess’s law states that:
Hess Law Worksheet PDF
Hess's law is stated as simply as enthalpy is a state function. Web hess's law worksheet author: Use a standard enthalpies of formation table to determine the change in enthalpy for each of these reactions. Web hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall.
worksheet. Enthalpy Worksheet. Grass Fedjp Worksheet Study Site
This law is a manifestation that. Web heat of formation worksheet. Calculator the table below contains information about four different reactions, labeled w, x, y, and z. Calculate δho for the reaction c2h4 (g) + h2 (g) → c2h6 (g), from the following data. You should try to answer the questions without referring to.
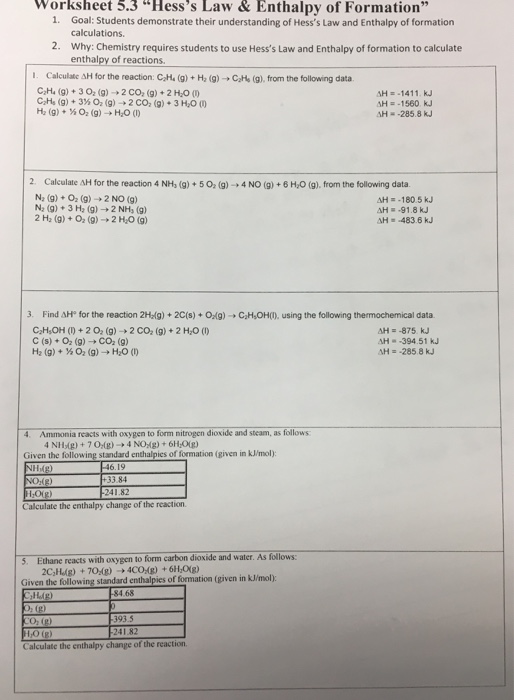
Solved worksheet 5.3 “ Hess 's Law & Enthalpy of Formation "
Web hess’s law can be stated as ‘the heat evolved or absorbed in a chemical process is the same, whether the process takes place in one or in several steps’. Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in.
Solved WORKSHEET HESS'S LAW NAME 1) Use Hess's law to
Web hess’s law states that: Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in one step or in a series of steps. Understand the relationships between heat, work, internal energy, and enthalpy. This law is a manifestation that. Calculate.
(CH 222 Lab) Hess’s Law Lab Report
Calculator the table below contains information about four different reactions, labeled w, x, y, and z. Understand the concepts of heat capacity, molar heat capacity, and specific heat. Web hess's law (worksheet) page id. No matter what way you go from reactants to products, you will end up with the same enthalpy of reaction for the reaction. To use hess's.
Hess Law Worksheet Pdf Thekidsworksheet
Web hess’s law, rule proposed by germain henri hess, stating that the heat absorbed or evolved (or the change in enthalpy) in any chemical reaction is a fixed quantity and is independent of the path of the reaction or the number of steps taken to obtain the reaction. Web hess's law states that if a process can be expressed as.
Hess S Law Worksheet worksheet
Write your answer to the ones place. This law is a manifestation that. Web hess’s law states that the enthalpy change for a chemical reaction is independent of the route taken. X = 100 then, (y+z) will also equal 100 units. You should try to answer the questions without referring to.
Hesss Law Worksheet - Hess's law worksheet created date: Hess's law is stated as simply as enthalpy is a state function. C2h6 (g) + 7/2 o2 (g) →. Calculate the δh for the reaction: C2h6 (g) + 3yz 02 (g) ~2 c02 (g) + 3 h20 (i) l1h =. Web know the first law of thermodynamics. To use hess's law, two principles must be understood: Web heat of formation worksheet. You should try to answer the questions without referring to. Work in groups on these problems.
Calculate h for the reaction 4 nh 3 (g. If you look at the change on an enthalpy diagram, that is actually fairly obvious. C2h6 (g) + 7/2 o2 (g) →. Use a standard enthalpies of formation table to determine the change in enthalpy for each of these reactions. Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in one step or in a series of steps.
One, if an equation is reversed, the sign of the δh value is also reversed. Calculate δho for the reaction c2h4 (g) + h2 (g) → c2h6 (g), from the following data. Web hess's law worksheet 1. Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in one step or in a series of steps.
Web Hess's Law Of Constant Heat Summation (Or Just Hess's Law) States That Regardless Of The Multiple Stages Or Steps Of A Reaction, The Total Enthalpy Change For The Reaction Is The Sum Of All Changes.
C 2h 4 (g) + h 2 (g) à c 2h 6 (g), from the following data: This means that the enthalpy change for the overall process will be identical regardless of. Write your answer to the ones place. Calculator the table below contains information about four different reactions, labeled w, x, y, and z.
For Example, The Enthalpy Change For The Reaction 2A + B → 2D Can Be Calculated Using The Enthalpies Of The Two Reference Reactions:
You should try to answer the questions without referring to. Understand the concepts of heat capacity, molar heat capacity, and specific heat. C2h4 (g)+ h2 (g)~c 2 h 6 (g), from the following data. Web hess's law worksheet author:
C2H6 (G) + 7/2 O2 (G) →.
Calculate h for the reaction 4 nh 3 (g. One, if an equation is reversed, the sign of the δh value is also reversed. Understand the principles of calorimetry. Web chem 12 hess’s law worksheet 1.
Web In Short, The Hess’s Law Is Based On The Fact That Enthalpy Is A State Function, And Therefore, Δh Rxn Is The Same Whether The Reaction Takes Place In One Step Or In A Series Of Steps.
\text {kj/mol}_ {rxn} kj/molrxn show calculator The total enthalpy change in a chemical reaction is independent of the route by which the chemical reaction takes place as long as the initial and final conditions are the same. C 2h 4 (g) + 3 o 2 (g) à 2 co 2 (g) + 2 h 2o (l) δh = − 1411 kj / mole c 2h 6 (g) + 7/2 o 2 (g) à 2 co 2 (g) + 3 h 2o (l) δh = − 1560. Web hess's law (worksheet) page id.